For help or advice with sample handling, contact Turner Scientific at 1-217-602-0306 or email: This email address is being protected from spambots. You need JavaScript enabled to view it.
Details of the methods we use - sample handling and dilution
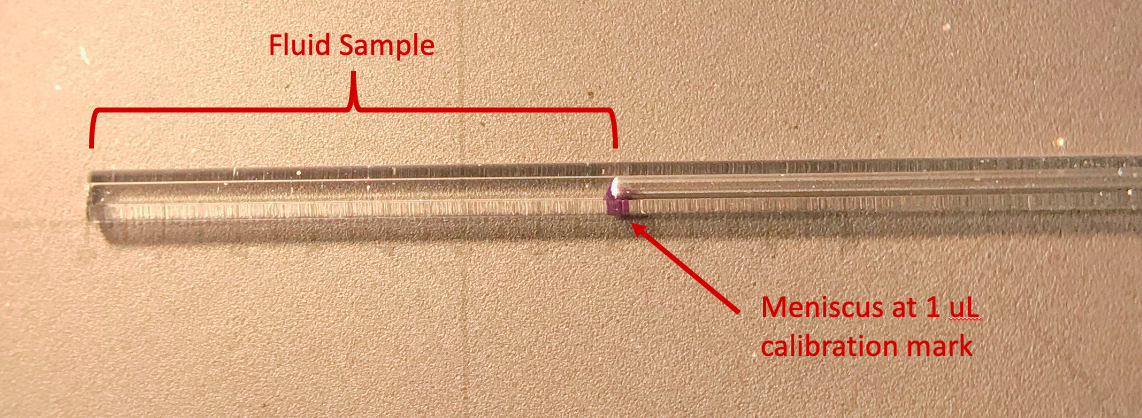
Perilymph is usually collected from the ear by allowing it to be pulled up, by capillarity, into a fine capillary tube. The sample volume is very small volume (0.5 – 5uL). It cannot be stored or shipped in the capillary tube as it immediately starts evaporating from the fluid surfaces exposed to air. With time, volume will slowly shrink as water evaporates even though the total drug content in the capillary will remain unchanged. We therefore measure the exact volume of the sample immediately after it is collected by measuring the length of the sample under a dissecting microscope with a calibrated eyepiece reticule.
The question is – how do we best handle such samples to measure perilymph drug levels by HPLC or spectrophotometer?
We prefer to transfer the sample to a vial with an air-tight sealed cap, as do most others. But the question is - what is the best way of doing that. While visiting other labs, we have come across a number of methods. We were interested in finding out which was the best one.
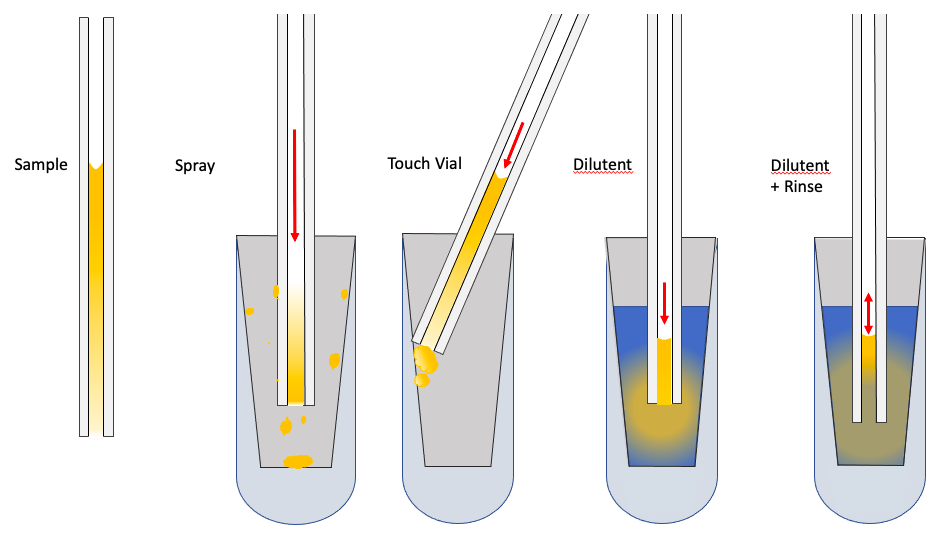
The methods in use by different groups include:
1) “Spray”ing the sample into the vial. In this procedure a pulse of pressure is applied to the back of the pipette (by mouth or by syringe) so that the sample is rapidly ejected into the vial. There it dries on the surface. When received by the analysis lab, a specific volume of dilutent will be added to the vial and concentration measured. This technique is often encouraged by the lab so that they can control the dilution and keep it similar to their calibration standards.
2) “Touch Vial”. In this case the tip of the capillary is placed in contact with the vial wall and gentle pressure is applied to expel the droplet onto the vial wall. Similarly, the droplet will evaporate and dilutent will be added to the vial by the lab performing the analysis. Again, this type of approach may be suggested by the analysis lab for their own technical reasons.
3) “Dilutent”. Here, a dilutent is first added to the storage vial and the sample is expelled into the dilutent. Typically for HPLC the dilutent is 25 – 50 uL of 50:50 methanol:water or 50:50 acetonitrile:water. For immediate spectrophotometry it can be up to 150 uL of phosphate-buffered saline. Sample vials are capped with tops including a silicone sealing ring so they can be stored and shipped with minimal volume loss. If the drug can be metabolized while in the vial, then the choice of diluent is very important. It should be "germicidal" and denature proteins that may potentially metabolize the drug, hence the use of methanol / acetonitrile.
4) “Dilutent + Rinse” (also known as the Hartsock method, since he perfected it). In this procedure, the sample is first expelled into the dilutent, then the applied pressure is reduced allowing dilutent to be drawn into the pipette, before being expelled again. The rinse is repeated 2-3 times in an attempt to transfer as much drug as possible from the pipette to the dilutent.
A Study Comparing Techniques
For years, we have used the “Hartsock” method. We were curious if the different methods made any difference to the outcome and whether the rinsing procedure we used had any influence. We therefore set up an experiment using solution with 20uM fluorescein as our standard. Samples of approximately 1uL in volume were taken and handled by the 4 methods. Every effort was taken to perform all the methods with the utmost care. In methods 1 & 2 (Spray and Touch Vial) 150uL of PBS was subsequently added to the vial before analysis. In addition, for the methods 1-3 (Spray, Touch Vial and Dilutent), the sample pipette was subsequently rinsed in a vial of 150uL clean dilutent, as a measure of how much drug was left in the capillary (shown as open symbols in the graph). Finally, the 1:150 dilution was replicated in a reliable manner by using a micropipette to add 20uL of fluorescein solution to 3 mL dilutent. These procedures were repeated both within the experiment and as 3 separate experiments performed on different days. Results were similar on each day and all results were pooled. No measurements were excluded from the study. In the graph below, all concentrations were normalized as a percentage of the concentration obtained by diluting with the micropipette.
Results
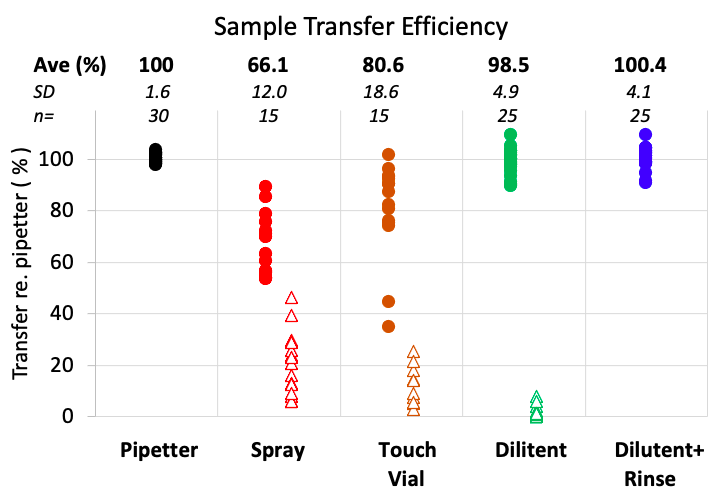
1) Spray: “Spraying” is an inefficient and highly variable method of transferring the sample from the capillary to a storage vial. On average, only 66% of the sample is recovered. Most of the missing portion remains in or on the capillary. An average of 20.4% is obtained by rinsing the pipette. The balance is probably lost as “mist”. The size of the storage vial used can play a role in the variability of this method, especially when the sprayed sample higher up on the vial wall may never be included when the dilutent is applied.
2) Touch Vial: This avoids the loss in the form of mist, but only 81% of the sample is recovered. Much, but not all, of the balance (average 12.2 %) is recovered by rinsing the capillary pipette.
3) Dilutent: In this method, 98.5% of the sample was transferred to the vial. Rinsing the pipette recovered an additional 1.5% (average).
4) Dilutent+Rinse: Using this method, an average of 100.4% of the sample was transferred to the vial, which is close to that obtained with a micropipetter
Conclusion
The Hartsock method is clearly a superior way to transfer samples to shipping vials for analysis. There is no need to include a correction for sample loss in handling. The “Spray” and “Touch Vial” methods both require substantial corrections for losses and will add considerable variation to the measurements. Even though the analysis lab may insist on/encourage methods that do not use dilutent, they need to know that it is a terrible way to handle perilymph samples and will cause massive problems with the data.